
Properties of Light

Separation of light and dark by Michelangelo (Sistine chapel, the Vatican)
There are many different types of light, red,
yellow, green,
blue, violet... In addition, there are many
types of light not visible to naked eyes: radio waves,
microwaves, infra-red light, ultra-violet light, x-rays, gamma-rays. To
see what kind of light we have at hand, we can break visible light into its
components by using a prism (or a diffraction
grating). This produces a spectrum.

One may thing all these are quite different. In fact, remarkable, they all have precisely the same nature. They are nothing but electromagnetic waves, the only difference being their wavelength.
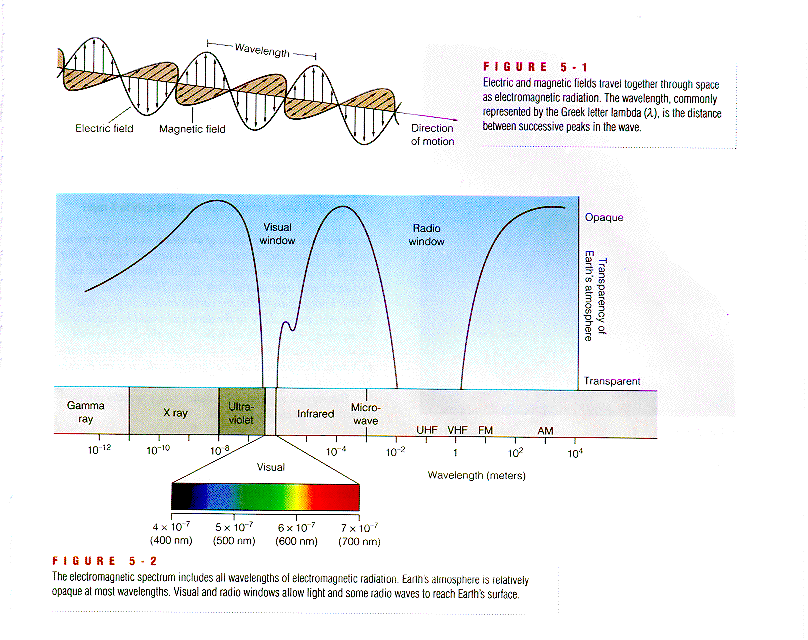
Having a wave character means that light is characterized by a wavelength L or a frequency f. All forms of light travel precisely at the same velocity: the speed of light
c = 300.000 km/sec,
which also defines the relation between wavelength and frequency
c=L*f.
Three Types of Spectra: the Fingerprints of Light
If we look at the colors of light coming from different sources, we can see several different spectra, as follows:
a) "Black-body" (continious) spectra - all colors found.
b) Discrete (line) "absorption" spectra - only several colors missing.
c) Discrete
(line) "emission" spectra - only several colors
found.

What is the origin of these
different spectra??? Let us take a closer look at these spectra and their
origin.
Radiation
from a heated object:
"Black body radiation"
Observing
the light spectrum emitted
by a distant object- such as our Sun- is an important diagnostic tool. For
example, we can determine the
temperature of the Sun in an accurate way by observing
its distribution of colors.
The main assumption behind this hypothesis is that
the temperature of an object (essentially how hot it is) is related, at the atomic level,
with the motion of the atoms it contains. The hotter the object the more rapidly the
atoms vibrate (or rattle). These rapid vibrations cause atomic collisions which are
responsible for exciting electrons from their ground state into some excited
states. Eventually, the electrons get de-excited an emit photons, or electromagnetic
radiation. This kind of radiation is called black-body radiation because
it refers to light emitted by an ideal object that is a perfect absorber or emitter of
radiation. Many astronomical bodies, including
stars, emit black-body radiation,
as if they were perfect absorbers/emitters.
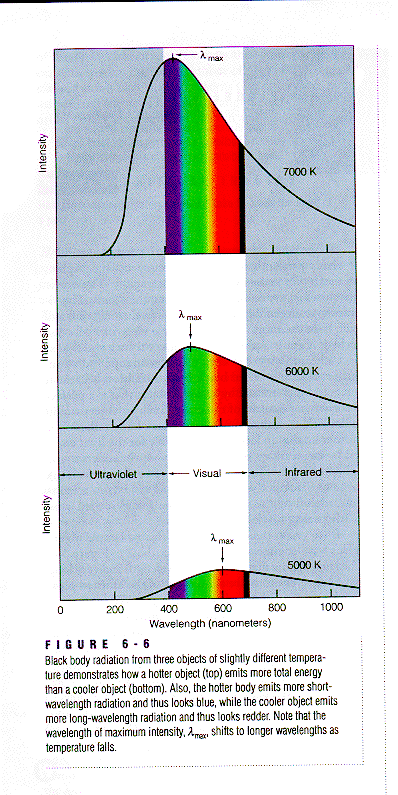
If you study the light emitted from our Sun - a typical star - you will observe an electromagnetic spectrum similar to one of the three shown in the above figure. Depending on the temperature of the star the maximum, or peak, of the distribution of light will be at a specific wavelength and color. For example, a star with a (surface) temperature of 5000 degrees Kelvin will have the peak of its radiation emitted at 600 nm - or in the yellow part of the visible spectrum. Remember that you can convert to degrees Kelvin - an absolute temperature scale - by simply adding the number 273 to the temperature in degrees Celsius. Computing the temperature of the object emitting the radiation is not difficult. You simply take the wavelength (expressed in nanometers) where you see the peak in the radiation spectrum (Lmax ) and compute the temperature T (in degrees Kelvin) by using
Wien's law: T=3,000,000/Lmax
Similarly,
if one knows the temperature
of an object - such as you - one can compute the wavelength at which
ones emitted spectrum will peak. For example, our external temperature
is close to the ambient temperature of, say, 27 degrees Celsius. To convert
to degrees Kelvin you simply add the number 273. Hence, a temperature of 27
degrees Celsius corresponds to 300 Kelvin. Then Lmax=3,000,000/300=10,000
nanometers = 10-5 m. This wavelength is in the infrared part of the
spectrum. Hence, your "body glow" is invisible to you and me - but probably not
to rattlesnakes!
Finally,
one last important formula.
You can compute the total energy radiated by a black-body every second and
per square meter of area by using the
Stefan-Boltzmann
Law: E
= s T4
As you may suspect, s is yet another constant - the so-called Stefan-Boltzmann constant - which is given by s=5.67 x 10-8 Joules/m2/sec degree4 . Hence, an object with a temperature twice as large as our Sun will emit 16 times as much radiation.
Atoms and Starlight
To understand
absorption and emission spectra, we need to learn a few facts about
atoms
producing them.
Matter is made out of atoms and there are over a hundred different atoms. Various atoms can bind together to form molecules and molecules bind to make complex compound. Because of their tiny size (only 10-10 meters) a quantity of a given substance can be observed with the naked eye only when it contains of the order of 1023 = 100,000,000,000,000,000,000,000 such atoms or molecules.
An
atom has a nucleus and
electrons
- which are moving in shells around the nucleus.
Thus,
an atom resembles a "miniature" planetary system with the nucleus playing the role of
the Sun and the electrons that of the planets. The nucleus is made of protons
and neutrons, which themselves are made of even smaller particles called
quarks. The protons have positive electric charge while the electrons carry
negative charge. Neutrons are electrically neutral (they carry no electric
charge). An electrically neutral atom is more stable when it has equal number
of electrons and protons. The attractive Electric force between the positively-charged protons in the nucleus and the negatively-charged electrons
binds the electrons and make them move in shells around the nucleus.

Atoms -
and therefore you and me
- are mostly empty space!
If you would take the atomic nucleus and
make it as large as a Basketball, then the innermost electron will
be located on the bleachers at Doak Campbell Stadium. The simplest
atom in nature is the Hydrogen
atom, having one proton, no neutron,
and just one electron revolving around the nucleus. The next simplest element is Helium and
the most abundant form of helium is the
isotope helium-4
having
two protons and two neutrons in the nucleus,
and two electrons.
Atomic excitations
One
of the main departures from
the solar system analogy of the atom is that, while planets can revolve around the Sun in any
specific orbit, the shells in which the electrons move in an atom are fixed and characterized
by an energy level; the lowest energy level is called the ground state. The fundamental reason
underlying this effect must be explained with Quantum
Mechanics
- a theory that even
Newton was not aware of - and one of the highest theoretical achievements of the 20th Century
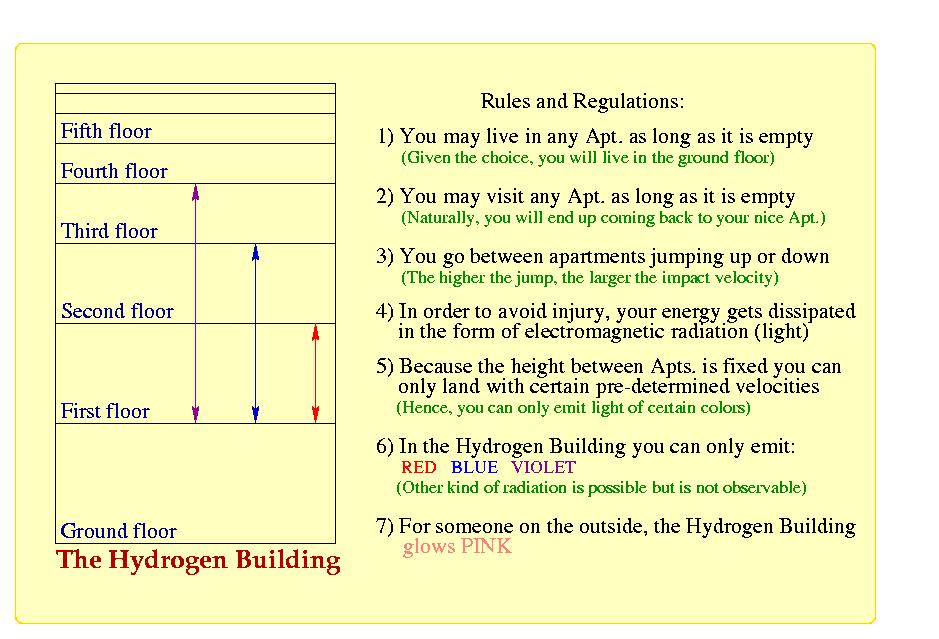
Physics. The permitted orbits (or shells) that electrons can occupy are like steps in a staircase
or apartment in a building; you can live in apartment one or apartment two - but definitely
you can not live in apartment number one-and-one-half. However, if an electron
in an atom receives energy from outside the atom, the electron can make a transition to an outer shell,
in the same way that you can take the elevator on the first floor (your orbit or shell) and
go to the third floor. Being in an outer orbit (or being in a higher floor) is refereed to being in
an excited state.
The electron stays in such an excited state only for a very short time and eventually
returns to its ground state by emitting
a photon (the quanta of light) which carries
the energy lost in the transition. A photon is a particle of light and at the same time an electromagnetic
wave.
Origin of
absorption spectra
Generally
the absorption spectrum
looks as follows. Photons
of only the proper wavelengths can be absorbed by the gas atoms and then
are re-emitted in random directions. Most of these re-emitted photons will not
reach the telescope and the spectrum will be missing some "colors".

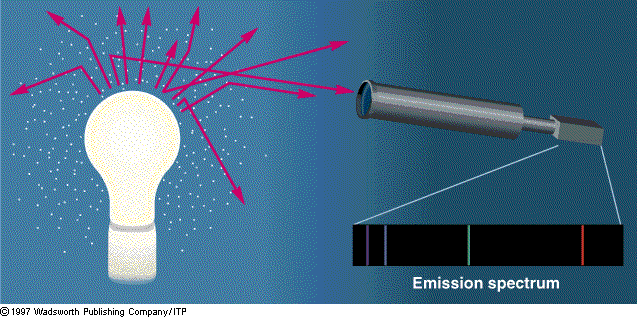
Origin of
emission spectra
Generally an emission spectrum is obtained as follows. Pointing the telescope away from the bulb, we can receive only those photons the atoms can absorb and then re-emit, producing emission lines in the spectrum.

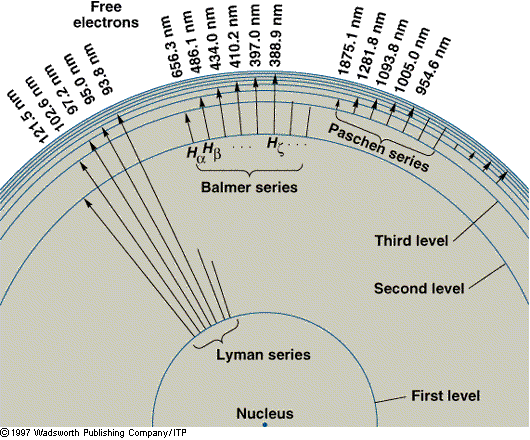
The Lagoon Nebula in Sagittarius is a cloud of gas and
dust about 60 light-years in diameter. Its gases (mostly Hydrogen)
are excited by the ultraviolet radiation of the hot, young stars
within,
and it glows in
the pink
- produced by the mixture of red (656 nm) the
blue (486 nm) and the violet (434 and 410 nm) Balmer lines.